HELPERBY'S SYNERGISTIC COMBINATIONS BEAT ANTIBIOTIC RESISTANCE
We develop approved anti-infective drugs into cost-effective combinations with synergistic anti-microbial activity to treat WHO Critical Priority Infections and reduce future antimicrobial resistance (AMR)
WHY HELPERBY SUCCEEDS WHEN SO MANY NCEs KEEP STRUGGLING
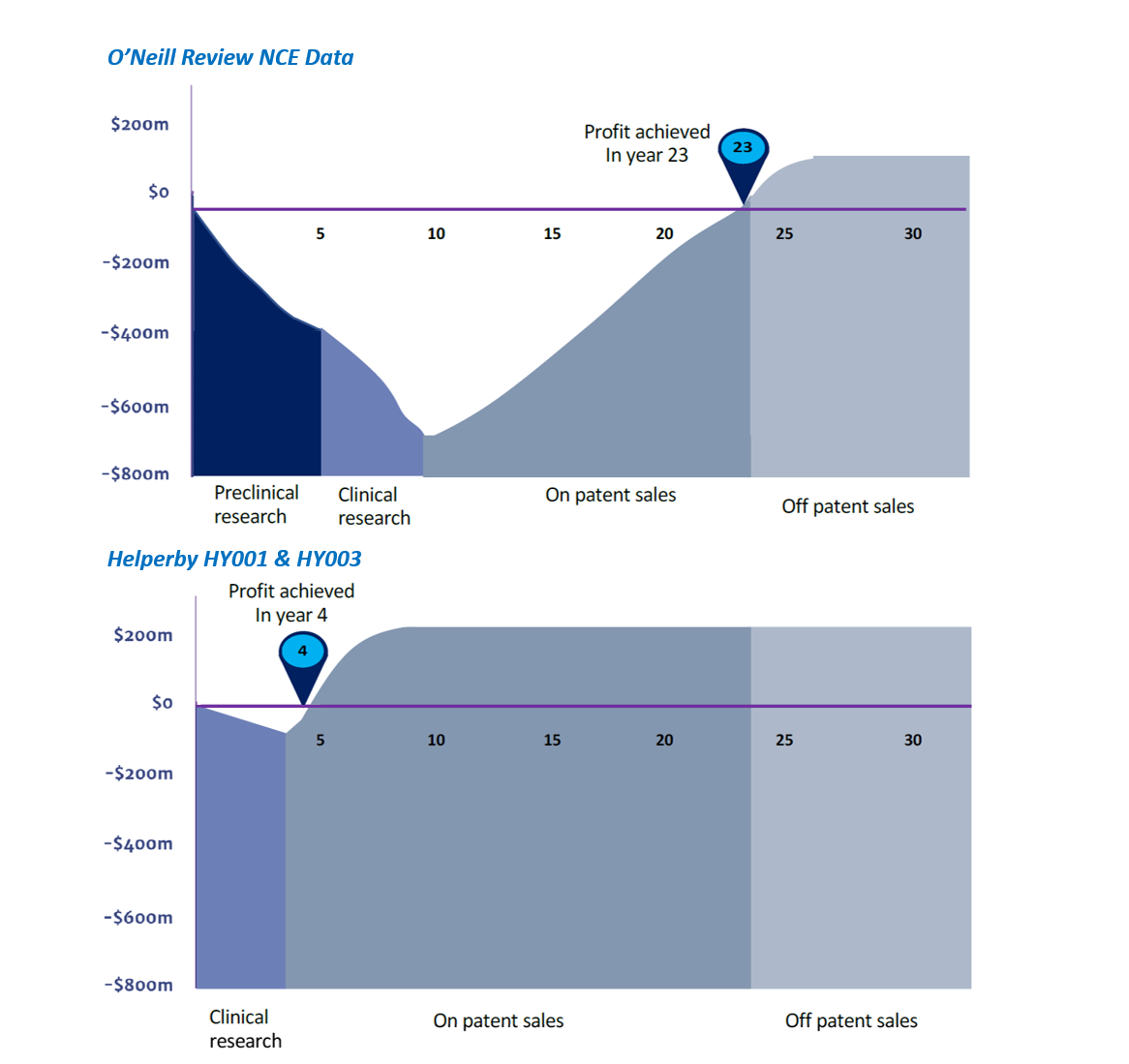
- Regulatory approval in Year 3 vs. Year 11 for New Chemical Entities (NCEs); giving longer on-patent and off-patent sales as sales life is not shortened by AMR (as all NCE antibiotics will be)
- Profitability in Year 4 vs Year 23 for NCEs, from > 10x lower costs, faster time to sales
- Implemented by an experienced team with proven business, commercial and development expertise in “virtual” business, avoiding unsustainable costs/time/risk of in-house infrastructure
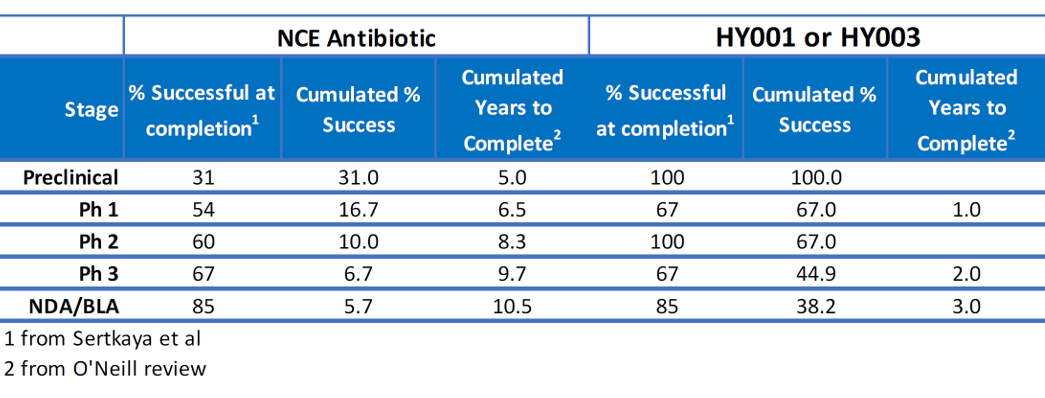
- Probability of success 6.7x higher than NCEs; approved drugs remove toxicology (69%) and Ph II clinical (40%) failure of NCEs(2), giving all stages at 67% success (as typical Ph III) for Helperby
WORLDWIDE INTERLINKED PROBLEMS
- Critical resistance of life-threatening pathogens growing worldwide, but poorly understood; O’Neill review (1) predicted AMR to cause 10M deaths/year by 2050; above cancer at 8.2M
- Too few new antibiotics, too narrow use AND continued AMR despite large R&D costs/time cannot keep pace with AMR developing by natural mutations in all bacteria
- Financial failure from unrecoverable costs vs. sales of NCE antibiotics from such limited use & life has significantly reduced investment
HELPERBY'S UNIQUE SOLUTIONS
- Reduce emergence of AMR by using combinations of two synergistic anti-infective drugs
- Treat life-threatening hospitalised patients in WHO Critical Priority indications
- Able to treat infections caused by Carbapenem Resistant Gram-negative bacteria, including strains with no effective treatment today, without causing future AMR
- Achieve profitability at acceptable price from reduced costs/risks of approved anti-infectives
PRODUCT HIGHLIGHTS
- Two different combinations of approved anti-infectives target complicated urinary tract (cUTI) and skin and soft tissue structure (ASSSI) infections in US and EU; each giving internal back-up to each other; strong competitive profile vs. current market leading products
- Unique products prevent AMR by known mechanisms, so broader, longer use-life achieved
- Both indications use global Ph III study supported by pre-clinical and in vitro microbiology data for US NDA by 505b and same EU filing routes; already proven by other combinations
FINANCIAL HIGHLIGHTS
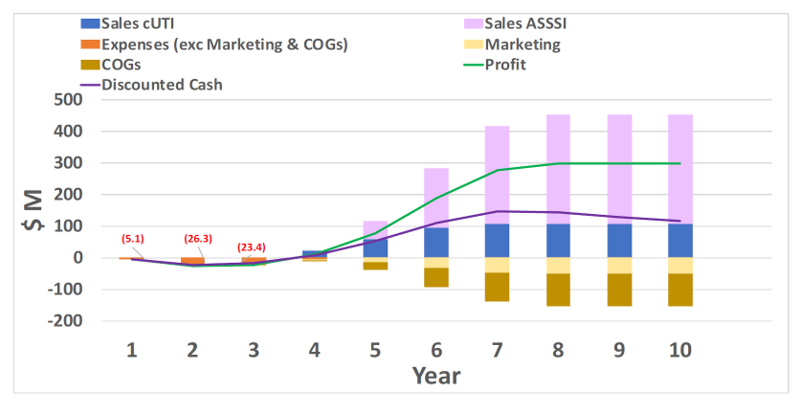
- Investment of $30M in Year 1; $20M in Year 2 achieves sales in Year 4; Year 10 discounted cash cumulates to $653M, giving well over 10x return
1) https://amr-review.org
2) Sertkaya A et al Analytical Framework for Examining the Value of Antibacterial Products; contracted to ERG by FDA Department of Health and Human Services 15 Apr ‘14

