Commercialisation
To discuss our latest funding round, please contact our investor relations team: investors@helperby.com
The potential to rapidly develop combinations to treat challenging infections
- Two different combinations of approved anti-infectives target complicated urinary tract (cUTI) and skin and soft tissue structure (ASSSI) infections in US and EU; each giving internal back-up to each other; strong competitive profile vs. current market leading products
- Unique products prevent AMR by known mechanisms, so broader, longer use-life achieved
- Both indications use global Ph III study supported by pre-clinical and in vitro microbiology data for US NDA by 505b2 and same EU filing routes; already proven by other combinations
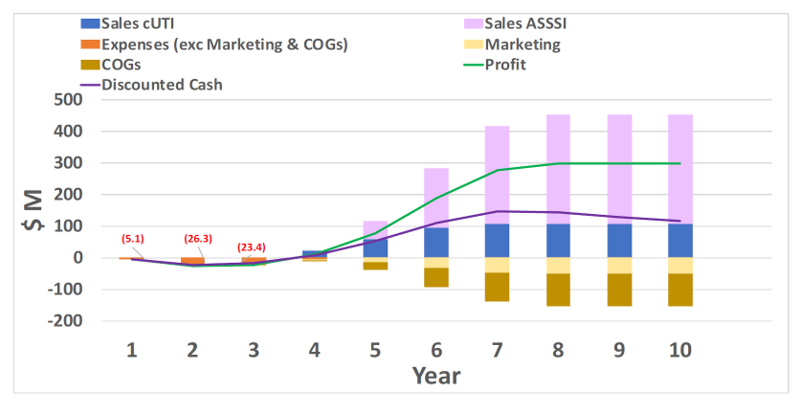
- Investment of $30M in Year 1; $20M in Year 2 achieves sales in Year 4; Year 10 discounted cash cumulates to $653M, giving well over 10x return